FDA warns J&J Covid-19 vaccine raises risk of rare neurological condition
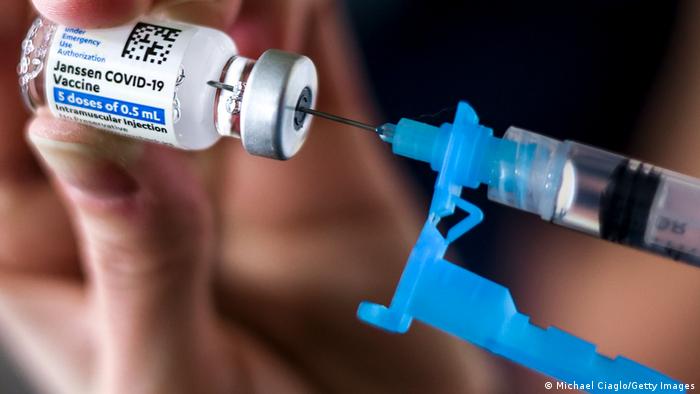
KATHMANDU: U.S. health regulators warned that the Johnson & Johnson JNJ Covid-19 vaccine is linked to a very small incidence of cases of a rare neurological disorder associated with other shots.
The Food and Drug Administration added the warning language to the J&J shot’s label on Monday, after finding a handful of cases of Guillain-Barré syndrome among the millions of people who have gotten the vaccine, according to a person familiar with the matter.
Guillain-Barré syndrome is a rare neurological disorder in which the immune system attacks nerves, causing temporary but potentially severe paralysis. The risk is a known one with vaccines, including some influenza vaccines and a leading shot to prevent shingles.
Johnson & Johnson said it had been in discussions with the FDA and other regulators about reports of Guillain-Barré following vaccination with its single dose coronavirus vaccine.
The company said that while the chance of such cases is very low, it exceeds the rate of normally reported cases among the general population by a small degree. The shot is a valuable tool in the fight against the pandemic, the company said.
“Evidence has demonstrated that Johnson & Johnson’s single-shot COVID-19 vaccine offers protection against COVID-19 disease and prevents hospitalization and death, including in countries where viral variants are highly prevalent,” the company said.
The Washington Post earlier reported the FDA’s plans to add the warning.
The warning would be the latest for a vaccine that federal health officials had cautioned raises the risk of a rare blood-clotting condition.
The risk of Guillain-Barré is about three to five cases per million recipients, the person said. The risk in the general population is about 1 in one million.
The Centers for Disease Control and Prevention said that 100 preliminary reports of Guillain-Barré have been detected after 12.8 million J&J doses were administered. It said such cases had largely been reported two weeks after vaccination and mostly in men 50 years and older.
-The Wall Street Journal










Facebook Comment